H2O2 (hydrogen peroxide) is a common oxidising agent and antiseptic. It exists as a pale yellow colourless liquid, but it is also solid and gaseous. People frequently confuse H2O2 with H2O, but this compound is not the same as the water molecule. When exposed to light, it has a bitter taste and becomes quite unstable.
Lewis Structure
Hydrogen peroxide (H2O2) has two O-H bonds and one O-O bond in its Lewis Structure. In addition, each oxygen atom has two lone pairs. The concept of the total number of valence electrons of oxygen and hydrogen atoms is used to draw the Lewis structure of H2O2.

Hybridization
Both Oxygen atoms are forming bonds, and as a result, these two Oxygen atoms hybridise.
One Oxygen atom forms a bond with a Hydrogen atom, a neighbouring oxygen atom, and has two lone pairs. In short, it must create four hybrid orbitals.
The steric number can also be used to determine the hybridization.
H2O2 has a steric number of 4, indicating that it is sp3 hybridised. To accommodate the lone pairs as well as the electron bonding pairs, one s orbital, and three p hybrid orbitals must be formed.
Molecular Geometry
Two Oxygen atoms form bonds with two Hydrogen atoms. Each Oxygen atom, however, has two lone pairs of electrons. According to VSEPR theory, these lone pairs try to repel each other, distorting the shape of the molecule.
The molecule tries to take shape and geometry in order to minimise repulsive forces between lone pairs of electrons. As a result, the geometry of hydrogen peroxide, or H2O2, is tetrahedral. The geometry of the molecule is tetrahedral because the atoms are not arranged in a single plane.
The AXN Notation method can also be used to determine the geometry of the molecules. The notation for H2O2 is AX2N2, which corresponds to tetrahedral geometry.
Bond Angle
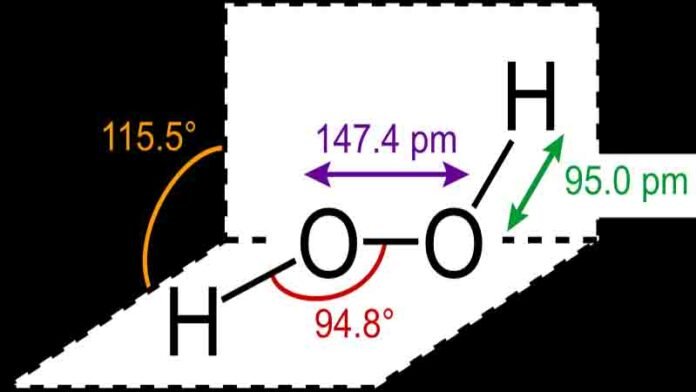
In general, the predicted bond angle for H2O2 for molecules with tetrahedral geometry and AX2N2 notation is 104.5°. However, two lone pairs of electrons in the molecule alter this bond angle slightly, resulting in H2O2 having a solid-state bond angle of 101.9° and a gaseous state bond angle of 94.8°.
When the state of the molecule changes, the bond angles change as the intermolecular forces between the electronegative Oxygen atoms decrease, and as a result, the bond angle decreases. As a result, the bond angles for H2O2 are 101.9° (S) and 94.8° (G).
Shape
Hydrogen peroxide is not a symmetric molecule because the repulsive forces between the lone pairs of electrons cause a distortion in the shape of the molecule. Hydrogen peroxide’s molecular shape is bent because the molecule is not linear and has a tetrahedral electron geometry.
As a result, H2O2 is a bent molecule.
Summary
Thus, the geometry of hydrogen peroxide can be concluded as-
- Hydrogen peroxide is made up of two hydrogen atoms and two oxygen atoms.
- H2O2 has a total of 14 valence electrons.
- There are three single bonds formed in the Lewis Structure of H2O2, two H-O bonds and one O-O bond.
- Each Oxygen atom has two lone pairs of electrons, so H2O2 has four lone pairs of electrons.
- H2O2 has sp3 hybridization because each Oxygen atom forms an sp3 hybrid orbital.
- In its gas phase, H2O2 has a bond angle of 94.8° and a bond angle of 101.9°.
- It has a bent molecular shape and tetrahedral electron geometry.